If you want the $25 term for it, you can say it is an oscillating electro-magnetic wave. That's pretty much a mouthful, but what does it really mean? Actually, the most important part of this definition is that light is a wave; it has wave-like properties. It, like sound or water, acts like a wave. Think of it as a wiggling, squirming thing if you like.
Since light does stuff like a wave, it has a certain size. You know
that not all waves are alike, and therefore not all types of light are
alike. You can distinguish the different types of light by measuring the
distance between consecutive peaks or consecutive valleys. This distance
is known as the wavelength. Often we abbreviate it with the
greek letter "lambda" - ![]() . Since it is just a distance, we can measure it in units
such as centimeters (cm), millimeters (mm) or other normal units of
distance.
. Since it is just a distance, we can measure it in units
such as centimeters (cm), millimeters (mm) or other normal units of
distance.
What else do we know about light? It travels. It has a certain speed, since it travels. The speed of light is denoted by the letter c, and is equal to 300,000 km/s = 186,000 miles/s. That's pretty fast.
Probably the most important aspect of light is that its speed in space is always the same - c is a constant. Actually, in some strange physics experiments people have been able to slow light down to very slow speeds, but those are under unusual circumstances and not what we see when we look up in the sky. As far as we're concerned, the speed of light is always the same! Don't forget that! Now, the speed of light doesn't care about the wavelength of the light; it can be long wavelength, short wavelength, or medium sized wavelength. In all cases the speed at which light travels is the same.
Take a look at Figure 1. There are two different types of light shown, each with its own wavelength. They both travel at the same speed. Let's say we are going to do another experiment with the light. We are going to watch the two types of light pass by and we are going to count the number of waves that pass by each second. We like doing these sorts of experiments, since we are strange scientists. Will we count the same number of peaks passing by each second? Here is a little animation showing the experiment - remember, both waves of light have to move at the same speed, regardless of their wavelength. This is another way to distinguish the different types of light. The one with more peaks squeezed together will give us a higher count, or you could say its peaks pass by more frequently, since both types travel at the same speed. In other words, you will count more peaks passing by each second for the shorter wavelength light. You could also say that it has a higher frequency compared to the longer wavelength light.
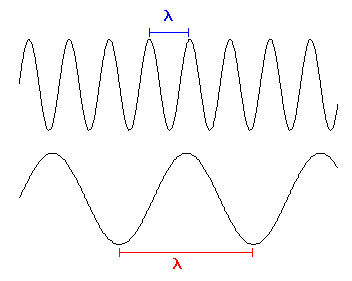 Figure 1.
Two different types of light are shown - one with a short wavelength and
another with a long wavelength. Or conversely a high frequency and a low
frequency.
Figure 1.
Two different types of light are shown - one with a short wavelength and
another with a long wavelength. Or conversely a high frequency and a low
frequency.
The other way to distinguish light is by the frequency, which is denoted by the letter f. Frequency is often given in units of "per second" such as "peaks that pass by each second." Generally it is harder to get a mental picture of light using frequency, so I'll just stick to wavelength to describe the different types of light.
Now there is a really neat relation between the speed, frequency and wavelength of light. Here it is -
c =f ![]()
This is pretty simple and straight forward (unlike some formulas). Since c must always have the same value, when you change the wavelength, the frequency must also change, but in the opposite sense. When the wavelength increases, the frequency decreases. Long wavelength light has a low frequency and short wavelength light has a high frequency.
As I mentioned, we'll distinguish the different types of light based upon their wavelength. It is important to be able to measure the wavelength to distinguish the various types of light since there are many different types of light, each with their own wavelength value. However, wavelength is often so small that it is difficult to describe in units as "large" as cm or mm. Often astronomers use the units known as ┼ngstroms, and in case you were wondering 1 ┼ = 10-10 m. These are pretty small units and are good at covering the range of wavelengths very well.
The following table gives the various types of light that astronomers study. Since frequency is a bit harder to picture, its value will not be given, though its size is denoted in general terms. The types of light listed in this table are all studied by astronomers, though for the most part a lot of astronomy is done in the visible part of the spectrum - what you can see with you eyes. The table is ordered in terms of wavelength from longest to shortest wavelengths. Don't worry about the last column; we'll get to that later.
| Light | Wavelength in meters | Wavelength in ┼ | Frequency | Blackbody Temperature (K) |
| Radio | 10-2 and greater | 108 ┼ and greater | low | 0.3 or less |
| Microwaves | 10-3 to 10-2 | 107 to 108 ┼ | 0.3 to 2.9 | |
| Infrared (IR) | 7 x 10-7 to 10-3 | 7000 to 107 ┼ | 2.9 to 4100 | |
| Visible | 4 x 10-7 to 7 x 10-7 | 4000 to 7000 ┼ | 4100 to 7250 | |
| Ultraviolet (UV) | 10-8 to 4 x 10-7 | 100 to 4000 ┼ | 7250 to 300,000 | |
| X-ray | 10-11 to 10-8 | 0.1 - 100 ┼ | 300,000 to 3 x 108 | |
| Gamma-ray | 10-11 and less | 0.1 ┼ and less | high | 3 x 108 and greater |
You have heard of all of these types of light in one way or another. Radio is just what you think it is - the type of light that your car radio picks up. The range of radio light is pretty wide, and the stuff that goes into your radio or tv is a pretty small part of the entire range. A lot of radio wavelengths are taken up by various communication systems, including military, CB radio, ham radio, and so on. Radio light is pretty much all over the place, even though you can't see it with your eyes. Another type of light that is vital to the existence of a typical college student is microwave light. Some people don't distinguish microwaves from radio, but we'll consider them as different types of light. Yes, they do cook your dinner in the microwave oven. However, most microwave light is harmless; a lot of cell phones use it for communication. The next type of light is actually the type given off by pretty much everything around you - infrared or IR. This is the type of light that is also associated with heat. You and all other objects around you are probably giving off IR light - things at room temperature do just that. You may have seen military or police videos that were shot using IR cameras - these are just picking up the light given off by humans and other warm things.
The type of light that you experience most of the time is visible light. You might just think of it as white light from a typical light bulb or the yellow light from the Sun, but if you were to take that light and pass it through a prism you'd get a rainbow. Visible light is made up of actually all sorts of different types of light (different wavelengths). It is often surprising to people to learn that white light is actually made up of all these colors combined together. If you have ever seen a rainbow then you have seen the full range of visible light. It is easy to remember the order of visible light from long to short wavelength since it is the same as the rainbow - Red,Orange, Yellow, Green, Blue, Indigo, Violet. Of course, if you don't have a rainbow handy, all you need to remember is Roy G. Biv, the world famous astronomer! Most pictures of objects that you'll see in this course were taken with visible light cameras, since we can easily produce those images and our minds are geared to interpret them. Red has the longest wavelength of visible light (it comes right after infrared, after all), while violet is the shortest wavelength visible light.
Continuing on down the light spectrum to shorter wavelengths, after visible light, or in this case after violet light, comes ultraviolet. Don't add an "n" to the name and make it ultraviolent, even though this type of light is rather nasty stuff. This is the type of light that gives you a sunburn in the summer and can lead to unpleasant things like skin cancer. Right on the heels of ultraviolet light comes an even nastier form, x-rays. You know this stuff is nasty when the dentist leaves the room when they x-ray your teeth. Finally, we have the shortest wavelength type of light that is studied by astronomers, gamma-rays. These are very short wavelength and are also rather rare. There is the whole range of light, from longest to shortest wavelength.E = hf
E = hc/![]()
Don't freak out; the formulas aren't that bad. Both formulas define the same thing but one uses frequency while the other uses wavelength. You first have the Energy (the E in the formula) of the light (or you could say the amount of energy in the photon) and it is related to the frequency - in fact it is directly related to the frequency. If you double the frequency, you double the energy. The other symbol in the formula, h, is a constant, and you can ignore it for the most part. You can also make the energy related to the wavelength as is shown in the second formula, since frequency is related to wavelength. Wavelength and frequency act in an opposite manner, so that when one goes up the other goes down, that's why the wavelength ends up on the bottom in the formula. All you need to remember are the following trends - Energy UP, Frequency UP, Wavelength DOWN. The reverse is also true. If you double the wavelength, the energy is cut in half as well as the frequency. If you triple the frequency, the energy is tripled but the wavelength is three times smaller. This whole thing relating the wavelength to the energy of the light kind of makes sense. Take a look at the table showing the different types of light. All of the nasty types of light are at the bottom of the table. This is the short wavelength end, as well as the high frequency end. What does that mean for the energy of the light - you got it, it's the high energy type of light, which kind of explains why some of it can kill you; it's nasty stuff!
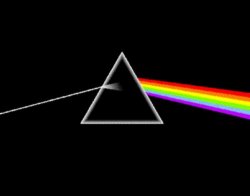 Figure 2.
The album cover from Pink Floyd's "Dark Side of the Moon" showing a
prism spreading out white light into the colors of the spectrum.
Figure 2.
The album cover from Pink Floyd's "Dark Side of the Moon" showing a
prism spreading out white light into the colors of the spectrum.
When we look at the spectra from various objects out in space, we don't see the same spectra - there are some with big gaps, some with colors missing, and some that are a real mess. How is this possible? What causes the different types of spectra?
To understand this, we have to understand how atoms interact with light and how spectra are produced. Perhaps this would be a good time to get a nice strong cup of coffee, or perhaps some highly sugar loaded caffeinated beverage - don't worry, I'll wait. First of all, you have to remember some basic physics about atoms. A typical atom has a specific structure based upon the type of atom it is. This structure includes many possible orbits for the electron or electrons if there is more than one. These orbits are located at various levels, which correspond to different energy levels. You might want to think of this as a bunch of mountain climbers. The climber with the most energy will go to the highest part of the mountain, up to a certain ledge, while those with lower amounts of energy will not get that high up, but will stay on the lower edges. If they get rid of their energy, they will go back down the mountain to the bottom level (known as the ground state in an atom). Electrons like to be in the ground state (the lowest level). You might want to think of them as mellow particles; maybe they just like to lay around the house all day in their underwear. Whatever the cause, they don't like to be excited and in those higher orbits (higher energy levels). If they do get into a higher orbit, they really want to get back down to the ground (lowest energy) state.
However, atoms are not exactly like mountains, since the electron can only go from one level to another if it absorbs or emits the exact amount of energy (light). It can't go to a place in between - there are only certain energy levels available to the electron - sort of like there are only certain places on the mountain where you can stop and stand safely. Electrons are very picky about the type of light (amount of energy) they will use. Only certain wavelengths of light will interact with the atom, since light is distinguished by wavelength. Let's say you have an atom with the electron in the lowest energy level (a hydrogen atom is the simplest to picture). Most wavelengths of light are ignored by the atom. If the exactly right wavelength comes along, the electron will absorb the energy (absorb the light) and the electron will bounce up to a higher orbit. The higher the energy of the light it absorbs, the higher the orbit it ends up in. Remember, it has to be the exact right type of light for this to happen. It will ignore all of the types of light that are wrong. When the electron moves to a higher orbit (higher energy state), we refer to the atom as being excited.
Electrons don't want to be in higher orbits; they like to be in a low energy state (mainly the ground state) - remember, they are lazy and like to have low energies. They want to get rid of any energy they pick up. To do this they give off the light needed to go down to lower energy levels. This light that they give off could be exactly like the light they absorbed or a combination of different types of light that add up to the same energy as the light that was originally absorbed. The end result is the atom gets rid of the energy it sucked up (it gives off the light in a random direction) and the electron is back down in the ground state. For a while the atom was storing up extra energy - this is when you would call it an excited atom - not because it won the lottery, but because it has extra energy in it. This scenario is shown in Figure 3.
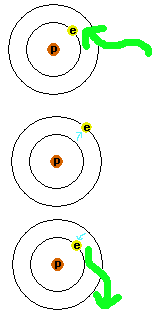 Figure 3.
An atom is shown with the electron in the lowest (ground) energy level.
Light of just the right wavelength is absorbed by the electron, causing
it to go into a higher energy level. As long as the atom has this extra
energy it is referred to as being "excited". To get back down to the
lowest energy level, the electron has to get rid of the energy and does
so by emitting light with the appropriate energy to make the transition.
Now it is just a plain unexcited atom again.
Figure 3.
An atom is shown with the electron in the lowest (ground) energy level.
Light of just the right wavelength is absorbed by the electron, causing
it to go into a higher energy level. As long as the atom has this extra
energy it is referred to as being "excited". To get back down to the
lowest energy level, the electron has to get rid of the energy and does
so by emitting light with the appropriate energy to make the transition.
Now it is just a plain unexcited atom again.
What happens if the energy the electron absorbs is too much? Is there such a thing as too much? You bet there is. The atom has a structure that allows for orbits up to only a certain energy level - sort of like the top of the mountain. If the electron absorbs too much energy and then goes up, there is no level that it can land in, since it has too much energy. This would be sort like having a rocket pack on your back as you climb a mountain, like what often happens to the Coyote trying to catch the Road-runner - he overshoots his target. The electron goes completely out of the atom. The atom is then said to be ionized; see Figure 4 for an illustration. Now the atom is sort of out of balance, since there was an electrical balance between the electrons and protons. With the loss of an electron, there is one less negative particle. For an atom like hydrogen, there is only one electron that can be lost. Other atoms have more electrons and can lose more and more, so long as the energy available is high enough to keep kicking the electrons out. Roman numbers are put behind an atom's name to distinguish ionized and unionized atoms. For example, regular, everyday, normal iron atom is refered to as Fe I. If it loses one electron, it becomes Fe II; lose another electron and it becomes Fe III, etc. If iron lost all of its electrons you would have Fe XXVII, since it has 26 of them. If the electron wants to get back together with the atom, or any other atom, it has to get rid of the energy it sucked up. Under the right circumstances it can give off light and then can go back to an atom.
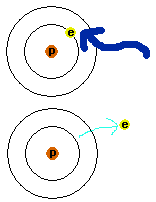 Figure 4.
An atom gets ionized, when light with high enough energy can knock the
electron out of the atom completely. The light is absorbed by the
electron so that it is able to escape from the atom.
Figure 4.
An atom gets ionized, when light with high enough energy can knock the
electron out of the atom completely. The light is absorbed by the
electron so that it is able to escape from the atom.
This is all exciting (yeah, right) but what about the spectra? Just a minute, I'm getting to that. Let's look at another situation. What if you have a bunch of atoms that are not getting bombarded by light but are just moving around a lot - they are perhaps in a container that is getting heated up. When you heat up gasses, the atoms move around faster. This is not good, since there will be a bunch of collisions. Does this mean their insurance rates go up? No, this means that there is a lot of energy of motion involved. You know what atoms like to do with energy, right? The electrons can take the energy of the collisions and change their orbits. To get back down to the low energy levels they have to give off light, so they do just that and go back down to their ground state. This is a situation where you can get light from a bunch of atoms without putting light into it in the first place; all you have to do is heat it up (technically, heat is energy, which is what light is, but it's not what you think of as light, right?). If the atoms are hot enough, they'll produce their own light. The atoms could be ionized or excited in this case, depending upon how fast they are moving and how much energy is involved in the collisions. See Figure 5 for the scenario.
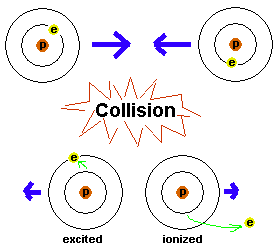 Figure 5. Two atoms head for a collision. The energy
of the atoms' motions is translated into getting the electrons bumped up
to higher energy levels. In one case, the electron just moves up to a
higher energy level and the atom is therefore excited. In the other
case, the electron is completely popped out of the atom, resulting in an
ionized atom. The electrons will have to eventually get rid of the
energy they have - by possibly giving off light - since they want to get
back down to the ground state.
Figure 5. Two atoms head for a collision. The energy
of the atoms' motions is translated into getting the electrons bumped up
to higher energy levels. In one case, the electron just moves up to a
higher energy level and the atom is therefore excited. In the other
case, the electron is completely popped out of the atom, resulting in an
ionized atom. The electrons will have to eventually get rid of the
energy they have - by possibly giving off light - since they want to get
back down to the ground state.
What about solid objects? When atoms get really, really close together where they are pretty much solid, or thick enough that they are pretty close to solid, then the levels of the atoms get really screwed up. They are sort of mushed around so that they aren't too picky about the type of light that they'll absorb - they'll absorb longer and shorter wavelength light and even longer and even shorter as the material gets denser and thicker. A solid can absorb pretty much all wavelengths of light, while something less dense, like a gas, will only absorb (or emit) certain types of light. A solid can also give off light at pretty much all wavelengths if it is heated up. For a strange reason physicists refer to a solid light source as a black body. Yes, that's right, an object that is giving off light because it is heated up is called a black body. This is in part due to the fact that such objects also absorb light, like how dark clothing is worn in cold weather to keep you warmer. There are a bunch of complicated formulas that define how "true" black bodies should behave. In reality, a perfect black body doesn't really exist in nature, but the rules that govern how a black body absorbs and emits light are useful in describing a lot of things, so it is often easier to just pretend that a lot of hot solid objects (like stars or heated up pieces of rock or metal) are black bodies.
Are you completely confused by now? If not, then you must have been skipping the really confusing bits. If you are confused, let's take a look at the rules that explain the various types of spectra. The ways in which light is absorbed and emitted by matter were studied and explained by a fellow named Kirchhoff, so we refer to these rules as Kirchhoff's Laws - these are the rules that explain how spectra are formed and define the different types of spectra.
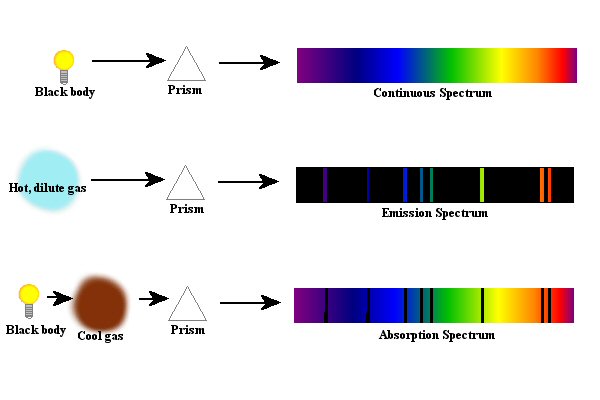 Figure 6. The
different types of spectra are shown - continuous, emission and
absorption.
Figure 6. The
different types of spectra are shown - continuous, emission and
absorption.
Figure 6 shows the various types of spectra. Often to denote a black body, a light bulb is used - remember, a black body is sort of a hot solid object like the filament in a light bulb. Again, it should be noted that there really is nothing in nature that is a true, perfect black body, but that some things do come close enough - at least close enough for us to use the formulas that describe black bodies. For the absorption spectra it should be noted that not all of the light that is absorbed by the cool gas cloud remains in the gas cloud. When the atoms in the gas cloud absorb the light, the electrons will be momentarily excited, but as you know, they don't like to be excited. Therefore, the energy that the atoms absorb does get eventually released, but the light is given off in random directions. The case of an absorption spectrum might be thought of as a "deflection spectrum" since the light is mainly just deflected off in random directions by its interaction with the atoms. In the case of the emission spectrum, something is causing the light to be produced by the gas itself - usually heat - and this causes the light to come out based upon the structure of the atom. By making the gas thicker and thicker, you would get thicker and thicker emission lines until you get to the point where the thing is solid and now you are looking at a black body and have a continuous spectrum.
In the case of Kirchhoff's laws 2 and 3, the different atoms in the gas give different patterns of lines corresponding to the type of light they absorb or emit. The type of light an atom absorbs or emits depends upon the specific structure of the atom (how far apart the energy levels are). In other words, a certain type of atom will produce a certain pattern of lines (dark absorption lines or bright emission lines) and each type of atom makes its own distinct pattern. An atom of hydrogen is different from an atom of helium, which is different from a carbon atom, which is different... well, you get the picture. See Figure 7 for a variety of spectra from some gases.
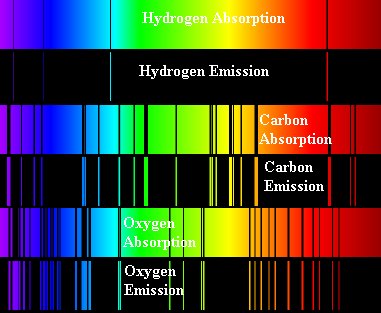 Figure 7.The
absorption and emission spectra for different gases are shown. Notice
how the spectra are sort of negatives of one another - what is absorbed
in one spectra for a particular element is emitted in the emission
spectra for that element.
Figure 7.The
absorption and emission spectra for different gases are shown. Notice
how the spectra are sort of negatives of one another - what is absorbed
in one spectra for a particular element is emitted in the emission
spectra for that element.
If the pattern produced by the emission or absorption spectrum is different for each element (and it is), then by looking at a spectrum, you can identify the gas that is involved in the production of the spectrum. This is just like fingerprinting the atoms - here's a method of obtaining element identifications! When astronomers try to determine the composition of a cloud of hot gas, a star or a galaxy, they use the spectra to provide information about the object. We don't even have to touch the star to figure out what it is made of, which is a good thing, since those critters are really hot. This is how astronomers can talk about what a distant galaxy is made of, though they have never had the chance to sample it directly - but just had the opportunity to look at its spectrum. In reality it is a bit more complex since objects are generally made up of a bunch of different elements - so all of those elements would be visible in the spectrum. In some cases it is even possible that the spectra have both absorption features and emission features - which is really complicated. An example is shown in Figure 8.
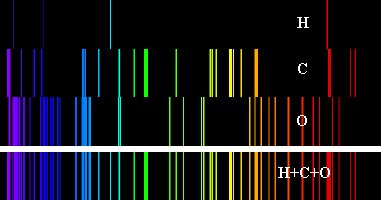 Figure 8. Emission
spectra for three elements are shown (Hydrogen, Carbon and Oxygen). If
all of these elements were in a hot gas cloud, they could produce the
spectrum at the bottom which is a combination of the three individual
spectra.
Figure 8. Emission
spectra for three elements are shown (Hydrogen, Carbon and Oxygen). If
all of these elements were in a hot gas cloud, they could produce the
spectrum at the bottom which is a combination of the three individual
spectra.
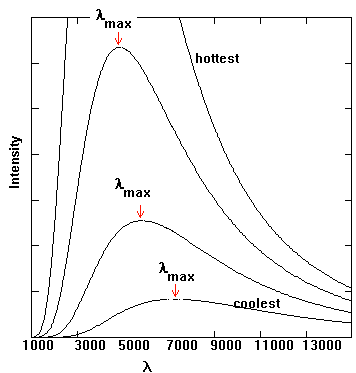 Figure 9.
Several energy output curves for blackbodies. The amount of energy given
off at different wavelengths is shown. Note how the curves all peak at
just one wavelength. This peak corresponds to the dominant type of light
that is given off by the black body.
Figure 9.
Several energy output curves for blackbodies. The amount of energy given
off at different wavelengths is shown. Note how the curves all peak at
just one wavelength. This peak corresponds to the dominant type of light
that is given off by the black body.
You might note that there is a peak for the energy output. The peak is at a different wavelength for the different temperatures of the black bodies. The higher the temperature, the shorter the wavelength of the peak. Hotter things produce mainly blue light, since short wavelength light is bluer, while cool things produce mainly red light. You may have seen this type of phenomenon while watching something heat up in a fire, like a piece of metal. First, the metal is dark and cool, then it is reddish, then orangish, then yellowish, then whitish-bluish - the hottest it will get. This color change is due to the change in the energy output peak - shorter wavelength for hotter things. Of course, very hot things could have their peaks in even shorter wavelengths, such as UV or x-ray, while very cool things could produce most of their light in wavelengths such as IR or radio. In these cases we would not be able to see with our eyes the main type of light that is given off, so it would look quite different to us. The relationship between the temperature and the peak wavelength (or color) is known as Wien's Law, and is given by this formula -
![]() max=
0.0029/T meters
max=
0.0029/T meters
where the temperature is in K and the resulting wavelength is in meters. The basic upshot of this formula is that when the temperature goes up, the wavelength where most of the light comes out at gets smaller (bluer). By using this formula it is possible to determine the temperatures of objects, or at least estimate them, so long as you can observe the peak for the energy output and you assume that the object is acting like a black body. Sometimes astronomers can do this, especially if the light comes out at a "normal" wavelength that we can detect. If you go back to the table listing all of the different types of light, the last column in the table refers to the temperature range needed to produce that type of light.
I should mention that the energy outputs of objects are quite diverse. The curves shown in Figure 9 indicate that energy is given out at many wavelengths, not just at the peak wavelength. If you can't see the energy at the peak wavelength due to a lack of the proper equipment, it is probably still possible to see the object - it may not look as bright as it would at its peak wavelength, but it should still be visible to some degree in the range of light that you can detect.E = ![]() T4
T4
where ![]() is a
constant and the energy emitted is the number of Watts per square meter
given off. This law is known as the Stefan-Boltzmann Law and is
primarily applied to stars, but can be used to estimate the output of
energy from most objects. A rather interesting aspect about
black bodies in general, is that the composition of the object
doesn't influence the energy output. Temperature is the only thing
that influences the energy output of a black body. You should make
note of the effect of changing the temperature in both these laws. As
the temperature goes down, the peak wavelength gets longer (goes up)
and the total energy output goes way down (that power of four on the
temperature is pretty strong!). What if you were to triple the
temperature? The peak wavelength would be three times shorter (1/3 the
original value), while the energy output would be 3x3x3x3 = 81 times
greater! A small change in temperature has a big effect on the energy
output!
is a
constant and the energy emitted is the number of Watts per square meter
given off. This law is known as the Stefan-Boltzmann Law and is
primarily applied to stars, but can be used to estimate the output of
energy from most objects. A rather interesting aspect about
black bodies in general, is that the composition of the object
doesn't influence the energy output. Temperature is the only thing
that influences the energy output of a black body. You should make
note of the effect of changing the temperature in both these laws. As
the temperature goes down, the peak wavelength gets longer (goes up)
and the total energy output goes way down (that power of four on the
temperature is pretty strong!). What if you were to triple the
temperature? The peak wavelength would be three times shorter (1/3 the
original value), while the energy output would be 3x3x3x3 = 81 times
greater! A small change in temperature has a big effect on the energy
output!
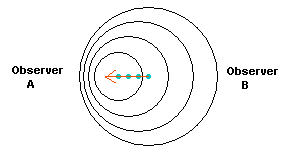 Figure 10. A light source is moving towards the left. The peaks
of the light waves are shown as circles that are moving away from the location they
where they were first given off by the light source. So the largest circle represents the first
light emitted by the source.
In the direction of its motion, the light waves are compressed, so that
Observer A would see the light source bluer than it actually is, while
Observer B would see the light source redder than normal (since the
light in that direction is stretched out to longer wavelengths).
Figure 10. A light source is moving towards the left. The peaks
of the light waves are shown as circles that are moving away from the location they
where they were first given off by the light source. So the largest circle represents the first
light emitted by the source.
In the direction of its motion, the light waves are compressed, so that
Observer A would see the light source bluer than it actually is, while
Observer B would see the light source redder than normal (since the
light in that direction is stretched out to longer wavelengths).
Astronomers describe the spectrum as being either red shifted or blue shifted. This just means that the light has been shifted due to, respectively, relative motion away from you or motion towards you. The degree of the shift depends upon the speed of the motion. You can determine the speed using this formula -
V =c ![]() /
/![]() ,
,
where V is the velocity (in km/s), c is the speed of light (=300,000
km/s), ![]() is the change in wavelength of the spectral features from the normal
wavelengths and
is the change in wavelength of the spectral features from the normal
wavelengths and ![]() is the normal wavelength. A larger shift results from a large velocity.
Figure 11 shows how the Doppler shift would cause the absorption
features in a spectra to appear at the "wrong" wavelengths.
is the normal wavelength. A larger shift results from a large velocity.
Figure 11 shows how the Doppler shift would cause the absorption
features in a spectra to appear at the "wrong" wavelengths.
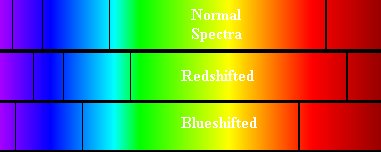 Figure
11. Absorption spectra shown
under three different circumstances. At the top, the normal non-moving
spectra with the features appearing at their appropriate wavelengths. In
the middle, the object is moving away from the observer (the person who
obtained the spectrum) so that the absorption features appear at
slightly longer wavelengths than normal. The bottom spectrum is the case
where the spectral source is moving towards the observer so the
features are blueshifted - occurring at slightly shorter wavelengths
than normal.
Figure
11. Absorption spectra shown
under three different circumstances. At the top, the normal non-moving
spectra with the features appearing at their appropriate wavelengths. In
the middle, the object is moving away from the observer (the person who
obtained the spectrum) so that the absorption features appear at
slightly longer wavelengths than normal. The bottom spectrum is the case
where the spectral source is moving towards the observer so the
features are blueshifted - occurring at slightly shorter wavelengths
than normal.
 Figure 14.
A refracting telescope operates by bringing light to a focus using a
lens or several lenses. The light collecting area of these types of
telescopes is limited.
Figure 14.
A refracting telescope operates by bringing light to a focus using a
lens or several lenses. The light collecting area of these types of
telescopes is limited.
Here's a good question - why do we want to bring light to a focus? Let's say you have a refracting telescope that has a 3 inch wide lens. All of the light falling in that 3-inch wide lens is being gathered by your telescope. How are you going to put all that light into your eye? Your pupil is pretty small, only a few millimeters wide at most. It's a waste of time if you can't get all of that light you are gathering into a convenient package that fits into your eye. The light needs to be gathered or focused so that it will fit into your puny little pupil. That's why we use the lenses to bend the light into a focus.
Unfortunately, there is a limit to the size of a refracting telescope, since the only support of the lenses is on the edges, just like eyeglasses. If a lens was very large, its weight would cause the glass to sag and the images produced by the refracting telescope would be distorted. Refracting telescopes had a size limitation that could not be overcome, at least not without sacrificing the light gathering area. Since size is so important (remember, size does matter), this is a pretty bad situation. One of the largest refracting telescopes is at the Yerkes observatory in Wisconsin (see Figure 15). The lens in this telescope is 40 inches wide - about as big as you can get.
 Figure 15.
Yerkes observatory refracting telescope. Note how long the scope is
compared to the amount of light that it gathers. This long length is
needed to focus the light properly. (Photo from the Yerkes virtual tour)
Figure 15.
Yerkes observatory refracting telescope. Note how long the scope is
compared to the amount of light that it gathers. This long length is
needed to focus the light properly. (Photo from the Yerkes virtual tour)
To overcome the size limits, another telescope design is usually used. If you have a curved surface which is highly reflective (like a mirror), the light could be brought into focus by having it bounce off the mirror at a certain angle. Since a mirror could be supported from behind, you can make a very large mirror! To focus the light, the best surface to use is a parabola (Figure 16), and this scheme is used in most large modern telescopes, not just those that you look through with your eye, but also radio, IR, etc. Currently most modern telescopes are of this type - reflecting telescopes.
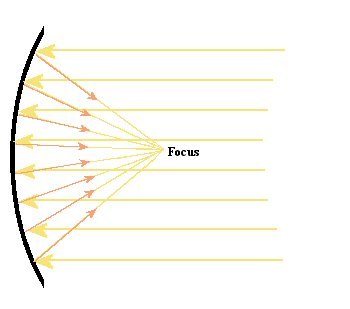 Figure 16.
Basic design for a reflecting telescope. The curved mirror is in the
shape of a parabola and this allows it to bring all of the light to a
focus in one location.
Figure 16.
Basic design for a reflecting telescope. The curved mirror is in the
shape of a parabola and this allows it to bring all of the light to a
focus in one location.
You might have noticed that the light in a reflecting telescope is focused at a point in front of the mirror. If you wanted to look at the gathered light, you would have put yourself in front of the mirror. This doesn't seem like a very logical way to observe objects, since your head would be blocking some of the light. Actually, with some telescopes, this doesn't really make much difference, since the size of the light gathering area is huge in comparison to the size of your head. Often instruments can be placed at the main focus location, or the light can be bounced around by other mirrors to take it to a more convenient location. This is shown in Figure 17.
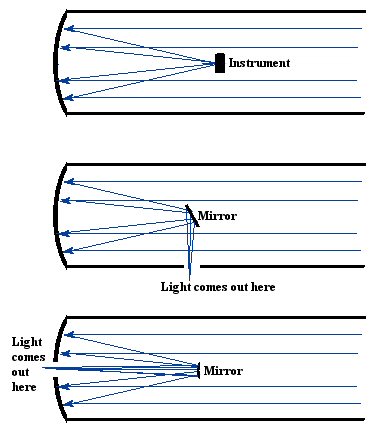 Figure
17. Different types of reflecting telescopes. Some are configured so
that instruments or even observers are located where the light is
focused, while other use mirrors to deflect the light to different
locations where instruments or astronomers are located.
Figure
17. Different types of reflecting telescopes. Some are configured so
that instruments or even observers are located where the light is
focused, while other use mirrors to deflect the light to different
locations where instruments or astronomers are located.
Visible light reflecting telescopes are currently limited only by the weight of the mirror and the ability to aim and support them. Also the amount of money available to build such large instruments and to keep them in working order. This last item may be the only real limit that we have now on the size of telescopes - the budget. Physical limits are overcome by a unique design, such as using not just one mirror, but a bunch of little mirrors that work together and mimic one huge mirror. Also, recent methods in making the mirrors themselves lighter has helped the process. Among the largest telescopes currently in operation or currently being built are the following -
The Gran Telescopio Canarias (GTC) is currently the largest single-surface telescope. Since making one giant mirror in not practical, this 10.4 meter wide beast is made up of 36 segmented mirrors that are placed together. And in case you are not too familiar with the metric system, 10.4 meters is equivalent to 34 feet. So this telescope's main mirror is a little bit wider than 34 feet. The telescope is located on the Canary Islands, off the coast of northern Africa. It is operated by a European astronomy group, and is usually only used by astronomers who work in Europe, so you may never have heard of this telescope. The size of the building that houses the telescope is just under 20 feet shorter than the Statue of Liberty in New York City - I'm not sure if that is something you are familiar with, but it is certainly higher than any grain silo in the state of Iowa.
 One of the 36 mirror segments that will go into the Gran Telescopio Canarias. |
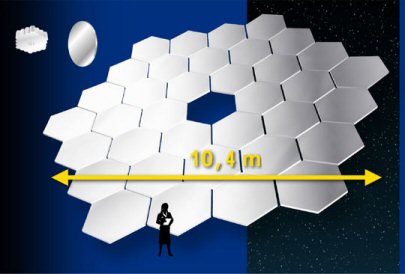 The schematic for the GTC, showing the overall width of the main mirror of 10.4 meters (34.1 feet) |
Keck telescopes - these telescopes are nearly as big as GTC at 10 meters. What is unique here is that there are actually two telescopes, each 10 meters wide. They have the same design as GTC and are located on a mountain in Hawaii.
 The Keck Telescopes. The two spherical domes contain the two telescopes that make up the Keck Observatory, the observatory to the left houses the Subaru Telescope. Image courtesy of the Keck Website |
 The main mirror of the Keck Telescope - multiple mirrors combined to act like a single mirror. Image courtesy of the Keck Website |
The Keck telescopes and GTC both use multiple pieces of glass to form the main mirror. The Large Binocular Telescope (LBT) is actually two mirrors, each 8.4 meters wide, and each made out of a single piece of glass. These mirrors work together, which effectively allows the collection of light to double.
Very Large Telescope - the winner of the "most unoriginal name in astronomy" contest. This is a big project where not one, not two, but four telescopes were built so that they can work together to mimic an even larger scope. Each of the scopes is 8.2 meters in diameter and when they are all working together, they operate as if they were a single 16.4 meter diameter scope. The VLT is in Chile.
 Large Binocular Telescope - here is a view of the LBT showing the two 8.4 meter mirrors. Image courtesy of NASA |
 Very Large Telescope - The 4 telescopes that make up the VLT are shown. Photo courtesy of the European Southern Observatory. |
 South African Large Telescope - The largest single telescope in the southern hemisphere. Photo courtesy of SALT. |
SALT - The names stands for South African Large Telescope. This one is designed like the GTC and Keck, so it is made of many mirror segments working together. It is about the same size as Keck, but is of course in the southern hemisphere, so that makes it the biggest single optical telescope south of the equator.
While these telescopes are relatively new, the old standard for a big telescope was 4 or 5 meters in size (13 - 16 ft). This used to be the size of the "big" telescopes available for research, but with new mirror design and manufacturing processes, it is possible that 10 meter mirrors will be built in the future. Actually, some people are even working on telescopes with 30 or more meter wide mirrors! Stay tuned for record breakers in the "biggest optical telescope" race over the coming years.
At UNI there are two observatories. Hillside observatory's telescope has a diameter of 16 inches, while the McCollum Science Hall telescope is 12 inches in diameter. They aren't 4 or 5 meters in size, but they are pretty nifty and relatively powerful instruments.
You may be wondering how you can have multiple telescopes working together; after all, astronomers can't look through both scopes at the same time, can they? Actually, they can. In the old days they just sat behind a single telescope and used their eyes to get a view of objects. Generally they had to be pretty good artists since that was the only way to record the information. They had to make sketches of everything that they saw. Some of the sketches are pretty good, which is rather impressive considering how cold, dark and difficult it is to draw sitting behind a telescope. Life for astronomers got easier around the late 1800s, when photography became the main tool for data collecting. Previously unseen, very faint objects were then detectable, since a photographic image could be made from a long exposure of the film. Long exposures are rather difficult to get, since you need to have your telescope aimed at the same object over the course of the evening, and since the sky has this rather annoying tendency to appear to move, it isn't easy. Probably more times than can be imagined, an astronomer would stay up all night taking a seven hour picture, only to have something come along and screw it up.
Nowadays, to increase the light gathering abilities of telescopes and to combine the data (light) from different telescopes together, and to make it easier to manipulate the data, electronic detection devices are often used. These are similar to the digital equipment that people have in things like plain old digital cameras, webcams and most video cameras. The most common type of electronic detector is a CCD, or Charge Coupled Device, which is really just a very light sensitive digital camera that translates images into a computer format which is easy to analyze or manipulate. It is also possible to take several different images from different locations and combine them with computers for a better image or to analyze the differences in the images. The only real disadvantage of CCDs is that they are generally pretty small and can usually only take a picture of a tiny part of the sky. Even small ones can cost several hundred dollars, while the biggest ones can cost several million dollars! Often when you see a modern astronomical picture of something in the sky, it isn't a photograph but a digital picture.
 (Right) One of the new CCD chips installed into
the Hubble Space telescope in 2002. Each chip is very sensitive to light
and can obtain high resolution, large scale digital images of the sky. Image
courtesy Hubble Space Telescope.
(Right) One of the new CCD chips installed into
the Hubble Space telescope in 2002. Each chip is very sensitive to light
and can obtain high resolution, large scale digital images of the sky. Image
courtesy Hubble Space Telescope.
There is a telescope project planned which will survey the sky using a 8.4 meter telescope in Chile. This project, the Large Synoptic Survey Telescope, will cover the entire visible sky each night by taking two pictures every 30 seconds. This will result in the creation of 36 gigabytes of data every 30 seconds. Read that sentence again - it said gigabytes. So in a few minutes enough data would be obtained to pretty much fill even large iPads. How can they obtain so much data? Well take a look at the camera they have - it is huge!!! And you thought your own digital camera took good pictures. This project is producing so much data that Google will be involved in helping to distribute and store it. The telescope is expected to be ready to go by 2014.
 (Left) A representation of the CCD chips to be used in the Large Synoptic
Survey Telescope project. Each of the 200 chips has the capacity to take a 16 mega-pixel image.
Image courtesy LSST Corporation
(Left) A representation of the CCD chips to be used in the Large Synoptic
Survey Telescope project. Each of the 200 chips has the capacity to take a 16 mega-pixel image.
Image courtesy LSST Corporation
 Figure 18. A
typical radio telescope. Light is reflected off the curved surface and
collected at the receiver at the top. This information is then fed into
a computer to produce an image of the sky. Notice that this is not a
solid surface, but a wire mesh. Photo by Seth Shostak from the Parkes
Radio Telescope (CSIRO).
Figure 18. A
typical radio telescope. Light is reflected off the curved surface and
collected at the receiver at the top. This information is then fed into
a computer to produce an image of the sky. Notice that this is not a
solid surface, but a wire mesh. Photo by Seth Shostak from the Parkes
Radio Telescope (CSIRO).
The largest single dish telescope is currently in Arecibo, Puerto Rico. This telescope is so large (300 m across) that it doesn't even move - it just sits in a valley and the rotation of the Earth allows it to view the sky. There is also some minor pointing ability with the focusing part of the telescope. Usually radio telescope dishes are 50-100 m in size. A larger telescope is under construction, the FAST, which stands for Five hundred meter Aperture Spherical Telescope. Construction should be finished in 2016.
 Arecibo Radio Telescope
Courtesy of the NAIC - Arecibo Observatory, a facility of the NSF. Photo
by David Parker/Science Photo Library.
Arecibo Radio Telescope
Courtesy of the NAIC - Arecibo Observatory, a facility of the NSF. Photo
by David Parker/Science Photo Library.
You have to remember that radio light is not something that your eyes can detect. Once the radio telescope has gathered up the radio light, it has to show you the picture somehow. This is where computers come in really handy. They can translate the signal into an image and also allow you to do some really nifty stuff with the data. Radio telescopes can be linked together over great distances - sort of like having stereo vision. This is known as interferometry and it provides greater resolution in the images. It is easy to combine signals, even if the telescopes are spread out over great distances, since all of the data that radio telescopes get are digitized. This results in very high resolution images. Radio telescopes can have resolutions that are 1/10,000 arcseconds, while visible light telescopes like the Keck or Gemini may have resolutions on the order of a 1/10 arcsecond. You can also use the interferometry trick with visible light telescopes, since they are becoming more and more digital-based.
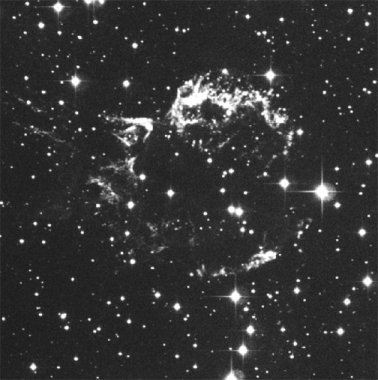 |
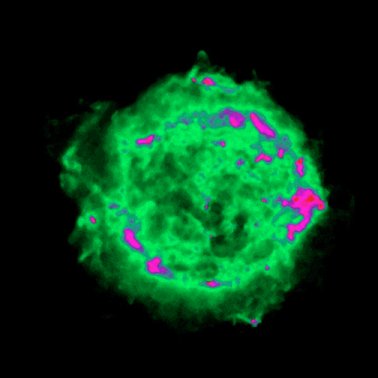 |
| Figure 19. Two views of the same object. An optical (visible light) image of a supernova remnant, the remains of a star that blew up, is shown on the left. The image on the right is of the same object, but taken with a radio telescope. This view shows more structure and the bubble shape of the gas cloud; however, it doesn't show the stars that are seen in the image on the left. This is because stars are generally not very strong radio light sources - they are usually too hot. Image from the Chandra website, NASA/CXC/SAO. | |
You'll want to take another look at Figure 19. The image on the left is what your eye can see, while the one on the right is a false color image based upon radio light intensities. This is similar to what you see on the local news when they show radar images of storms. In those images the maps are color coded to show the location and concentration of rain, or snow or something similar. In astronomical images from data that our eyes can't see (like most types of light), you are seeing usually a concentration or intensity of light. The actual color of the object isn't being shown, so don't expect there to be large clouds of blue, red and green material floating out in space. It is also likely that images taken with visible light telescopes are manipulated to show certain features, and they can also be enhanced in artificial ways. So basically most modern astronomy images are "altered" in various ways to help astronomers learn specific details.
A large group of radio telescopes that work as if they were one large dish is found in New Mexico at the VLA (Very Large Array- another one of those really exciting telescope names). In this case, there are 27 individual radio telescopes that can act like a single large one. Currently an even larger group of telescopes is being built in Chile. Eventually there will be 66 individual radio telescopes for the Atacama Large Millimeter/submillimeter Array (ALMA). And plans are underway to build radio telesope arrays covering even larger areas. The Square Kilometer Array will be made up of thousands of telescopes, all working together, and creating a total light collecting surface of one square kilometer. But that will take a while to build.
 The Very Large
Array (VLA)- Photo courtesy of Dave Finley, National Radio
Astronomy Observatory and Associated Universities, Inc.
The Very Large
Array (VLA)- Photo courtesy of Dave Finley, National Radio
Astronomy Observatory and Associated Universities, Inc.
 Atacama Large Millimeter/submillimeter Array-
Photo courtesy ESO/B. Tafreshi (twanight.org)
Atacama Large Millimeter/submillimeter Array-
Photo courtesy ESO/B. Tafreshi (twanight.org)
You may have noticed something about the telescopes shown so far - a
lot of them are located in rather remote locations, especially the
visible light telescopes. This is because astronomers are so annoying
that people can't stand them - no, that's not the real reason. Actually,
to really study the sky, you need a really good view of it. The best
possible view is one away from cities, preferably in a place where there
are few cloudy skies and low humidity. That's way there are so many
observatories located on tops of mountains, since the humidity is
pretty low up there many times and there is less atmosphere to look
through. Amongst the best sites in the world for having observatories
are in the southwest US (Arizona and New Mexico), the top of the
mountain Mauna Kea in Hawaii (which is a hopefully extinct volcano), the
mountains of Chile and the desert regions of Australia and many other
countries. Radio telescopes aren't so picky; they can be found in more
locations, since radio light isn't as sensitive to things like city
lights as visible light it, though you often need a large area for a
radio telescope.
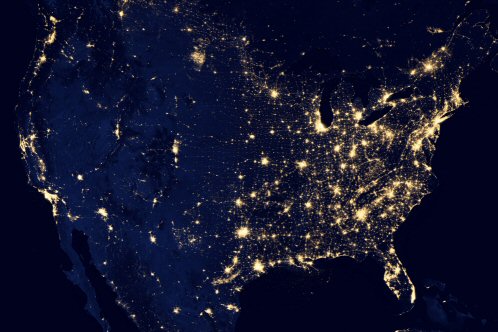 Figure 20. An image of the USA at night as seen from
space. The light seen is due to city lighting for the most part. Of
course, all of this light seen from space is wasted electricity - why
would someone want to light up the sky? This also shows why astronomers
would want to put a telescope in a place like Arizona or New Mexico
where there is less light pollution. If you click on the image you'll
see the full sized view.
Figure 20. An image of the USA at night as seen from
space. The light seen is due to city lighting for the most part. Of
course, all of this light seen from space is wasted electricity - why
would someone want to light up the sky? This also shows why astronomers
would want to put a telescope in a place like Arizona or New Mexico
where there is less light pollution. If you click on the image you'll
see the full sized view.
Radio and visible light are only two types of light that exist. What
about the others wavelengths of light? How do astronomers study them? We
would like to study them, but there is this rather annoying thing known
as the atmosphere. The atmosphere prevents other types of light from
getting to the surface of the Earth, and it also has the annoying
tendency to limit the quality of our view since it can blur stuff quite
a bit. Pretty much all of the other types of light are blocked out at
sea level. It is possible to detect some of the wavelengths of infrared
light at very high elevations. Apart from that, you can't detect any
ultra-violet, x-ray or gamma-ray light sources from the surface of the
Earth. How do astronomers study those types of light? We have to get
above the atmosphere - we have to send telescopes into space.
There have been many satellites that have been used by astronomers, but
we'll look at only the "biggies" here. Except for the gamma-ray and
x-ray telescopes, the telescopes use the same old parabolic mirror
design to gather light. Of course, since the telescope is in space, the
images are obtained generally by using digital cameras or a similar
detection device, so the data can be easily translated into a format
that our eyes can handle.
The main infrared satellite up there is Spitzer (launched 2003), however it has run out of coolant, so it is limited in its ability to measure some of the lowest energy IR light. A recently launched IR satellite is WISE, which is a survey telescope, similar to IRAS. It was launched in December 2009, but its primary mission lasted only lasted until 2011. During that short time it did find may low temperature objects including some of the coolest stars. WISE has continued in a new mission, called Near-Earth Object WISE to search for things that get close to the Earth, as the name implies. This new mission doesn't require the super cooled systems that were previously used, but it has been able to find 34,000 new objects. Some IR astronomy work has shifted to the tops of high mountains, since it is also possible to do some IR astronomy work in that format, and it is cheaper than a satellite. Click here to see a comparison of a region in space where stars are forming. Such regions have a lot of cool gas and dust, and the stars tend to be rather cool, so they show up better at IR wavelengths than at visible wavelengths. Note how some stars are only seen in the IR image. These two images are from the Hubble Space Telescope (courtesy of STScI/NASA).
There aren't any general mission UV satellites currently in orbit, or satellites that are
designed to investigate mainly UV light sources. Often UV satellites have specific purposes, for
example, galaxies, the Sun, or interstellar material.
Ultraviolet telescopes tend to look at very hot objects, things like ultra hot stars or stellar explosions are popular objects as well as material heated up to extreme temperatures around stars.
Chandra (1999-now) is a current x-ray telescope that is in orbit. Its views of the sky show astronomers how various high energy objects, like active galaxies and supernovae, are behaving. Also the NuSTAR mission (2012-now) will be looking for very high energy x-rays, possibly indicating the presence of black holes.
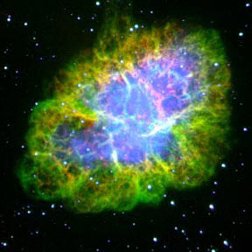 |
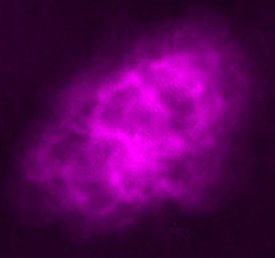 |
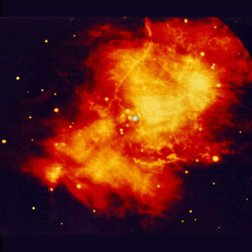 |
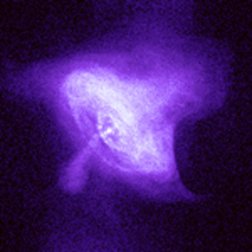 |
| Images of the same object taken with four different telescopes. In the upper left is the optical (visible) light telescope image of a cloud of material from a star that exploded about 950 years ago. In the upper right is the same object, but as seen with a radio telescope. The lower left shows an infrared view of the object, while the lower right shows the x-ray view. Even though this is the same object, different features are seen, or not seen, depending upon which telescope is used. Images from the Chandra website, courtesy of Palomar observatory, NRAO, W. M. Keck observatory and NASA/CXC/SAO. | |
 The Hubble Space
Telescope (HST) - image courtesy NASA, STScI. Many of the
pictures in this course are from this telescope. You can check out many
of the images if you visit the HST
website.
The Hubble Space
Telescope (HST) - image courtesy NASA, STScI. Many of the
pictures in this course are from this telescope. You can check out many
of the images if you visit the HST
website.
While it is not necessary to put a visible light telescope in space, the need for one is obvious. The atmosphere has a tendency to blur images, so by placing a telescope above the atmosphere, the clarity of the images is increased a great deal as well as the ability to gather more light. The currently largest optical telescope is the Hubble Space Telescope - HST (1990 - ). While the mirror of the HST is only 2.5 meters in diameter, its location in space makes it a very powerful tool. Soon after it was launched it was discovered that the telescope's big mirror was not in the correct shape so that the light did not focus. To give you an idea of how picky these telescopes can be, the error in the HST mirror was 0.000002 meters. Even though this is a pretty small amount, it really screwed up the telescope. It had to be fixed in space. A later space shuttle mission went up to fix it and now it works just dandy. Recently a new camera was also installed, which will allow it to take even better pictures than before. The HST is by far the most annoying telescope ever built! Why do I say that? Mainly because of all of the new things it discovers (and I have to keep re-writing my notes!). Recent repair missions have extended the life of the HST, hopefully until we can replace it with an even bigger visible light telescope. The HST may be able to keep working until 2030-2040, which would be really amazing. The telescope that is planned to replace the HST is the James Webb Space Telescope, which is scheduled to be launched in 2018. But until it is up there, we don't have to worry about it (and I don't have to revise my notes again).
Future Telescopes - Right now there are plans on making much larger and obviously much more powerful telescopes. As previously mentioned there are plans on making visible light telescopes that are larger than 10 meters in diameter, and even plans for the next version of the Hubble Space Telescope. While bigger telescopes may be better, there are other ways to improve images of objects, especially when viewed from the Earth where the atmosphere causes so much blurring. The latest idea is something called adaptive optics. This technique involves compensating for the blurring effects of the atmosphere by altering the light that is collected so that it is not as smeared out. This can be done in a variety of ways including, having telescopes change their shapes to compensate for the atmospheric effects. In some cases the images obtained in this method are better than those obtained by the Hubble - take a look at the images shown below to see what I mean. Expect not only bigger telescopes in the future, but also much more sophisticated methods of dealing with the light - whether that involves different collecting techniques or analysis techniques, we'll just have to wait and see.
 Image from the Hubble Space Telescope showing a bright star and surrounding stars. Photo courtesy of the Hubble Space Telescope (STScI/NASA). |
 Image from the Very Large Telescope taken using Adaptive Optics. There are more stars visible in this view and each star is clearly seen - not blurry - since the telescope is not only larger than the Hubble, but used adaptive optics. Photo courtesy of the European Southern Observatory. |