I am always tickled by this low tech approach to one of our compounds. The reaction is shown below
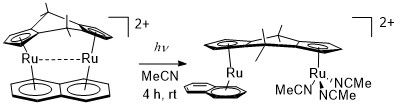
The naphthalene complex absorbs in the visible region to “unhinge” the naphthalene ring. The resulting compound allows us entry into all sorts of different diruthenium complexes since the acetonitriles and naphthalene are now very labile.
So, to do the photolysis we need a light source. The biggest compact fluorescent bulb that I could find on the internet

We usually photolyze about 200 mgs of compund at a time. We split that amount into 6-8 NMR tubes to allow the photolysis go a lot faster. The fan is to keep the samples cool during the photolysis period. We need a holder for our NMR tubes. To the untrained eye it looks like just a piece of cardboard with holes punched in it. The pieces of tape on the cardboard allows us to keep the hole snug enough to hold the NMR tubes.

Here is the final set up. Some compressed air also helps keep things cool.